Liquid Metals: Stretchable Barriers, Conductive Fibers, and Microchannel filling
- khashayar Ghaffarzadeh
- Jun 12, 2023
- 3 min read
Updated: Jun 13, 2023
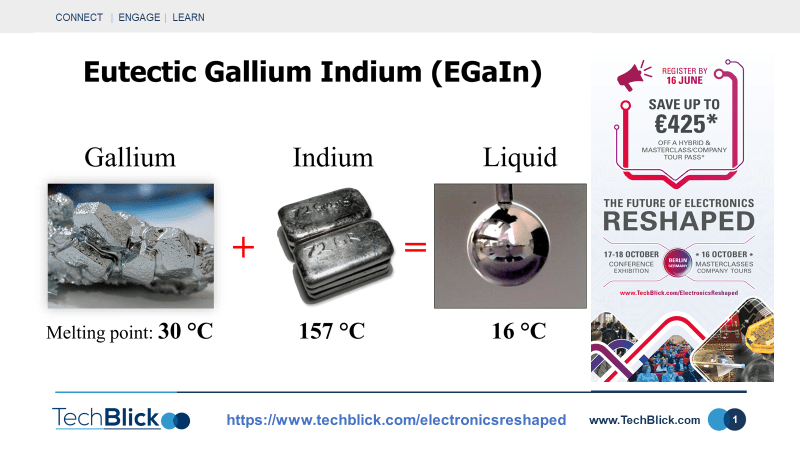
Liquid metals are extremely versatile. They are composed of GaIn liquid [Ga and In have melting temperatures of 30C and 157C]. The material has no vapor pressure and has viscosity like water, but with metallic properties.
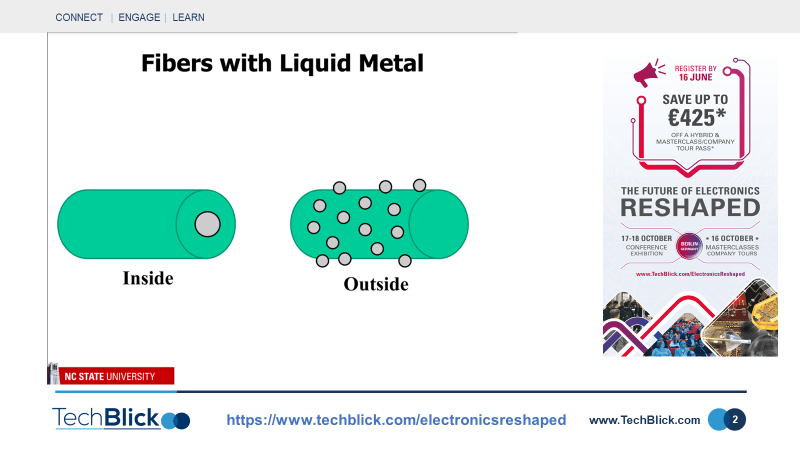
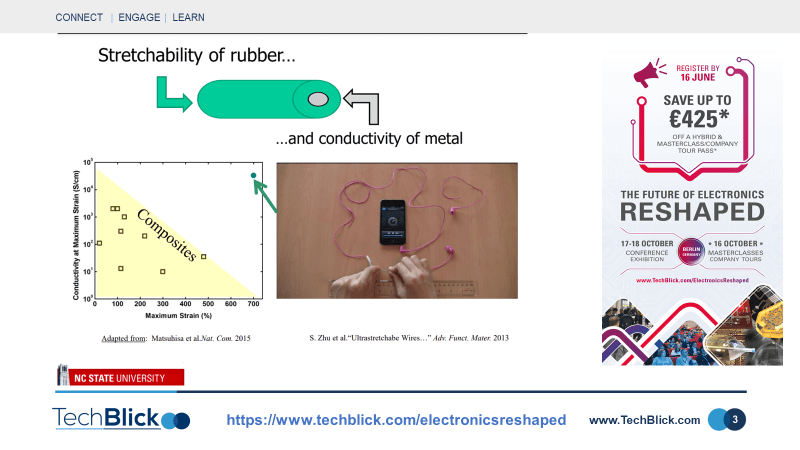
Dr. Michael Dickey from North Carolina State University recently presented interesting results at TechBlick, showing self-healing structures, conductive fibers, stretchable barriers, and filled microfluidic channels using liquid metals. These liquid metals almost instantaneously form a thin [few nm thick] non-conducting GaOx passivating layer which stabilises the structure. The oxide layer can easily be broken [with mechanical force] to form a conductive pattern. As can be seen in slide [2], these materials can be applied to fibers in two ways: filling the fibers/tube or adding the particles to the outer surface of the fiber/thread. In the first approach, a material can be formed with the elasticity of rubber and the conductivity of metal. As shown in slide [3], the conductance changes with strain [up to 500% demonstrated]. Note that the conductivity of the liquid metal itself does not alter with strain, but the cross-section of rubber fiber changes, thus changing overall conductance. As shown in slide [4], to apply these materials to the outer surface, a droplet suspension of liquid metal nanoparticles [100 nm to a few micrometer in diameter] needs to be formed through, for example, sonication. The droplets become stabilized thanks to the presence of the oxide layers again. As shown in slide [5], the fiber can then be dipped several times into the solution and dried. This way, textile threads covered fully with a networking of liquid metal nanoparticles can be formed. At this point, the network of liquid metal nanoparticles is non-conducting due to the presence of oxides. However, with the application of a simple mechanical force, it can be made conductive. These technologies can enable the surface of the textile to be used as the base, for example, for deposition of copper layers. This is a novel approach, although mechanical and thermal stability of the liquid metals on the fibers remains an open question.
Join us and the global community in Berlin on 17-19 OCT 2023 to RESHAPE the Future of Electronics - explore the world-class agenda, masterclass programme, and expo floor here https://www.techblick.com/electronicsreshaped
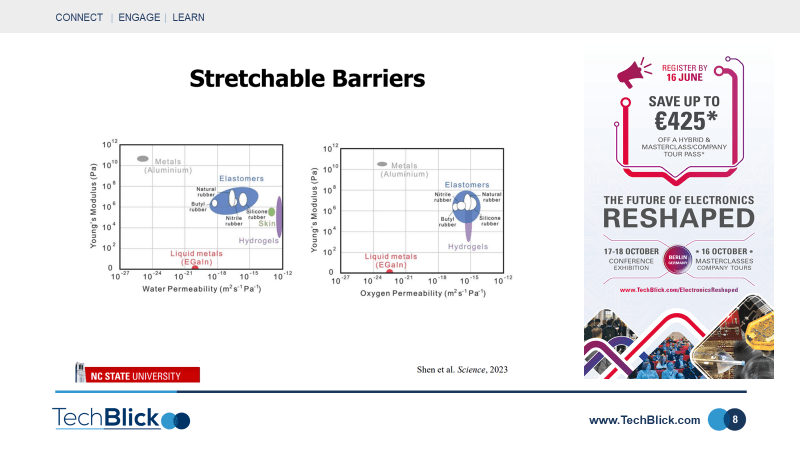
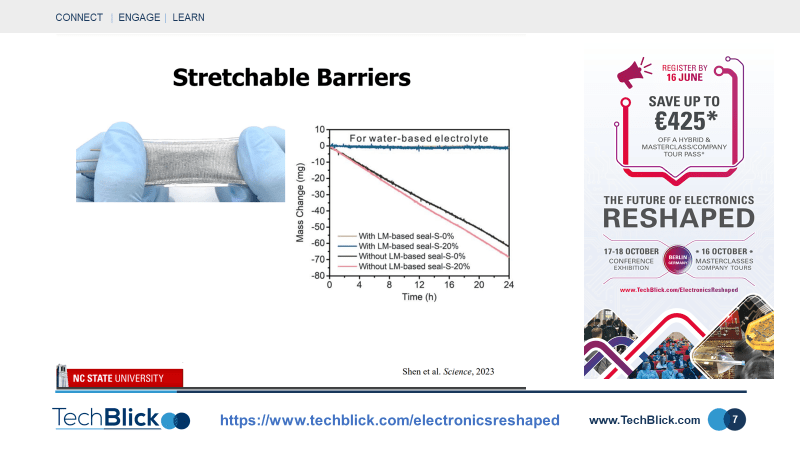
Slide [6] shows an interesting application: vacuum filling liquid metals. Here, the polymer material includes microfluidic channels. The entire system is under vacuum, thus the channels are under vacuum too. The liquid droplet is placed atop the structure. At this point, pressure is reintroduced, pushing the liquid metal through the microfluidic channels. This is a unique capability as filling such microchannels is not easily achievable with other materials such as nanoparticle inks or particle filled pastes. Slie [7] shows one of the most interesting applications of liquid metals. Traditionally, polymers are used as packaging but they have poor barrier properties. To compensate, a thin metal coating is generally applied. This improves barrier properties, but renders the hybrid structure [metal+polymer] rigid. A novel solution is to apply liquid metals to the polymer structure, achieving booth good barrier and elasticity properties. This is shown in slide [5], which shows that Liquid Metals can have good permeability [not as good as metals but comparable] whilst not compromising the stretchability of polymers. To demonstrate the utility of this, a stretchy pouch battery was made. As shown in slide [8], the battery with liquid metal based seals, unlike those without liquid metal seals, report no change in mass as a function of time even when stretched. This is an interesting use case in that it opens a new technology for stretchable barrier technology.
Do NOT forget to join the Free-to-Attend Festival on 22 June 2023
https://www.techblick.com/innovation-festival-june-2023

















Yorumlar